¡Monoclonal antibodies, ¿who decides the names of each one?
– Part 5!
It helps to look at the letters backwards
October 8 de 2024
¿Can a drug be called whatever it wants? The issue is not that simple. So ¿who decides what a drug or therapeutic agent is called? The WHO.
In the case of monoclonal antibodies (mAbs, or Moabs), the INN is also applied, and they are assigned a generic name based on a specific schematic structure.
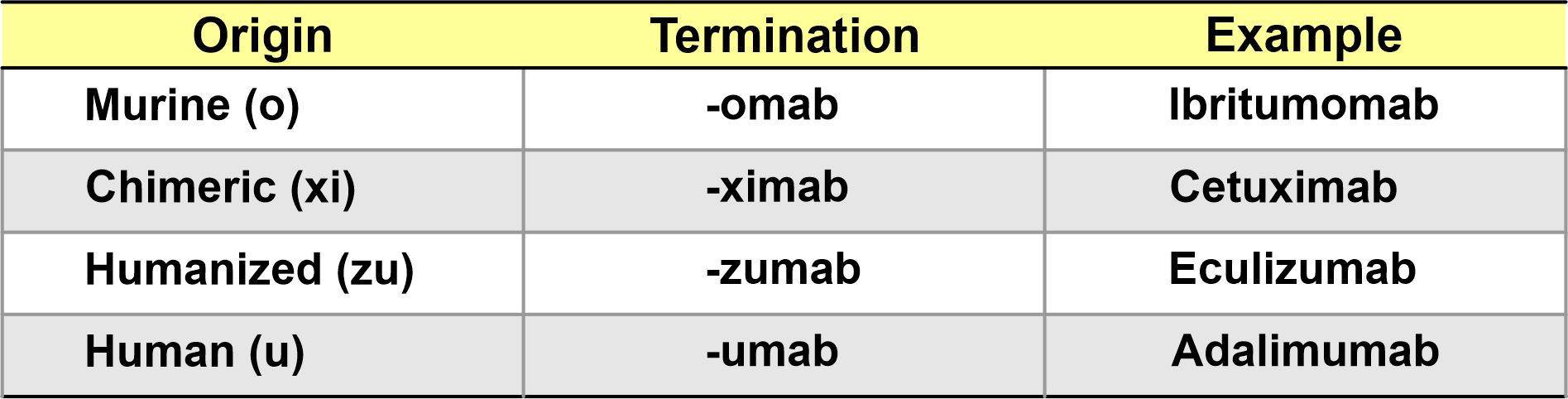
In the past, the situation was much simpler than it is now. Until 2017, all mAbs ended in –mab. The INN or name of each mAb was assigned according to a simple scheme that contemplated 4 aspects:
a) Random prefix: Free choice by the manufacturer
b) Type of target: Which target is it directed at (tumor cell, bacteria, endothelium, vascular cell, etc.)
c) Species of origin: Animal, chimeric, humanized, or fully human.
d) Common suffix: mab, but due to the explosion in the number of mAbs, this has changed.
At the moment, there are already more than 800 mAbs registered, with all kinds of origins, technologies, objectives, etc. However, the scheme initially developed for their names remains, although it has been expanded in a gigantic way.
